What do we mean by ‘highly excited’ molecules? These are molecules that have ~ 35,000 cm-1 of internal energy (remember that a hydride stretch fundamental has ~ 3000 cm-1 of internal energy). This is enough energy to break chemical bonds. How do we do this? We use a laser to transfer ground state molecules to excited electronic states. Furthermore, since we are interested in dynamics, we want to know precisely how much energy the molecule has when it undergoes reaction. Since room temperature molecules have a lot of energy in them, we expand them in a vacuum to a few Kelvin, essentially removing all internal energy from the molecules.
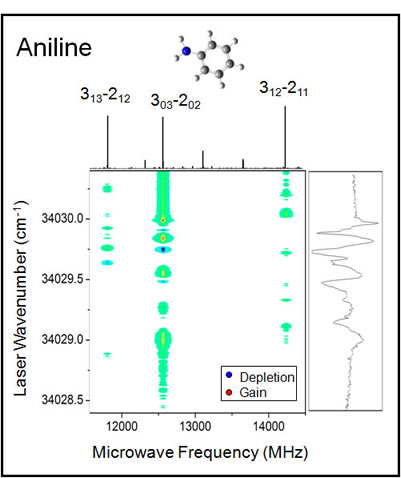
To monitor ground state depletion of a molecule, we focus on a particular molecular transition observed in our rotational spectrum. For aniline, our experiment focused on three different ΔJ =2→3 transitions. The laser was scanned over the S1←S0 origin band while the intensity for the specified transition was monitored. As the laser became resonant with the rotational transition, the population difference between the energy levels changes resulting in a change in intensity of the molecular signal. A dip in signal corresponds to population moving from the lower level whereas a gain corresponds to a movement from the upper level.